Soluble Silicates: Properties, Manufacture, Analysis & Safe Handling for Welding Consumables Production
Soluble Silicates are the “glue” that turns dry flux powders into a workable paste and, later, a strong, clean-burning coating in welding consumables. In covered electrodes and submerged arc welding fluxes, alkaline silicate solutions—typically sodium or potassium silicate (“water glass”)—provide adhesion to the steel core,strength for handling, and stable rheology for uniform extrusion or coating. Potassium silicate is often preferred where easy arc striking and steady ionization are desired, while sodium silicate can improve early strength and cost efficiency. Small additions of organic modifiers (e.g., cellulose derivatives, alginates, dextrin) tune viscosity, thixotropy, and water retention, helping powders wet out without segregation.
This article explains soluble silicates (sodium and potassium) in plain terms: they’re made by melting sand with soda or potash, then dissolving the glass in water. We show how to describe a grade (by solids %, density, and the silica-to-alkali ratio) and what that means in use. Key behaviors are simple: solutions are alkaline, get thicker with more silica/solids, and get thinner when warmed. You’ll find easy lab checks (basic acid titrations and a weigh-and-ignite method), plus clear tips for delivery, storage tanks, piping.
Handy charts link viscosity to temperature and density to help set process conditions.
The article ends with how WESPEC helps manufacturers use silicate binders in welding electrodes and submerged arc welding fluxes—from formulations and equipment choices to QC, training, and start-up support.
- Definition
- Manufacture
- Properties
3.1 Structure
3.2 pH value
3.3 Density
3.4 Viscosity
3.5 Specific heat
3.6 Melting point
3.7 Drying
3.8 Freezing
3.9 Chemical reactivity - Analysis
4.1 Titration of alkali
4.2 Titration of silica
4.3 Gravimetric determination of silica - Packing, Transport and Storage
5.1 Packing
5.2 Storage tanks
5.3 Piping and valves
5.4 Pumps - Safe Handling of Silicates
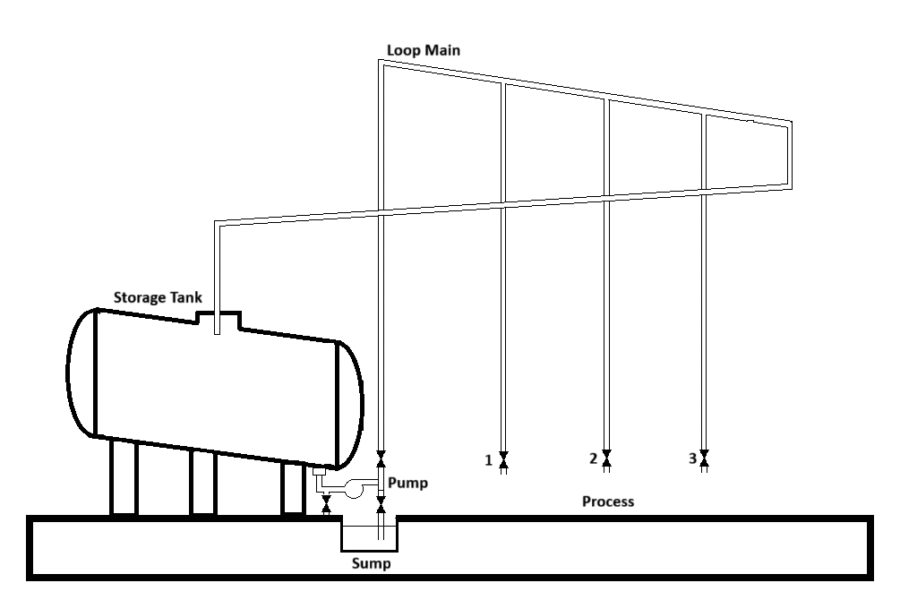
- Layout of the Head Tank Distribution System
- Layout of the Loop Main Distribution System
- Change of Viscosity with Density at Constant Sio2: Na20 Ratios
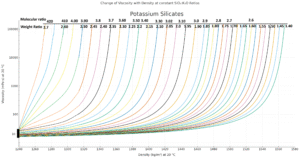
- Change of Viscosity with Density at Constant Sio2: K2o Ratios
1.Definition of Soluble Silicates
Soluble silicates are products made by fusing quartz sand and soda ash or potash. The lump glass which is obtained from the molten silicate is dissolved to solutions of varying composition and concentration.
Plant for the production of soluble silicates / Soluble silicate glass in lumps

The composition of the soluble silicates are conveniently indicated by the solid content and the density as well as the weight ratio of percent silica (SiO₂) divided by percent alkali metal oxide (Na₂O or K₂O), without implying neither that silica or alkali metal oxide exist as such in the systems nor that the ratio is determined by any stoichiometric limits. Thus, it is not usual to write formulas to define commercial silicate solutions.
For example, a sodium silicate solution containing 26.8% SiO₂ and 8.0% Na₂O would be said to have a weight ratio of 3.35.
The molecular ratio is obtained as follows:
- Sodium silicate: molecular ratio = 1.032 × weight ratio
- Potassium silicate: molecular ratio = 1.568 × weight ratio
The sodium and potassium silicates having a weight ratio exceeding 2.50 are classed as “neutral” and those with a weight ratio less than 2.50 are termed as “alkaline”. However, this classification is incorrect because in actual fact all silicates have an alkaline reaction.
2.Manufacture of Soluble Silicates

Soluble silicates are made by the high temperature fusion of silica sand and soda or potash in oil or gas fired furnaces at about 1300°C. The molten silicate is drawn continuously onto a slow moving steel tray conveyor where the glass solidifies on cooling and cracks into glass-like lumps.
The silicate lumps differ from ordinary glass in that they are soluble in water, especially at elevated temperatures and pressure. Under normal conditions the lump glass has a low dissolving rate in water.
3.Properties of Soluble Silicates
3.1 Structure
Alkali metal silicates form aqueous solutions in which the silicate anions range from monomeric orthosilicate to highly polymerized species. The average rate of polymerization depends on the silica to alkali ratio and the concentration of the particular silicate solution. The polymerization of the silica anions increases in proportion to the rise of the ratio and the solution becomes more viscous.
3.2 pH value
Although the terms “neutral” and “alkaline” for the description of silicate solutions are quite common all sodium and potassium silicate solutions have an alkaline reaction. The pH values of the commercial grades vary from 11 to 13. A dilution with water at the rate of 1:10 to 1:100 does not change the pH of the silicate solutions considerably: the 1% solutions show a pH between 10 and 12.
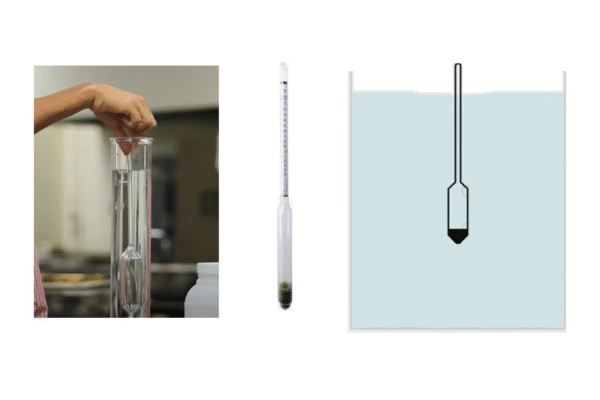
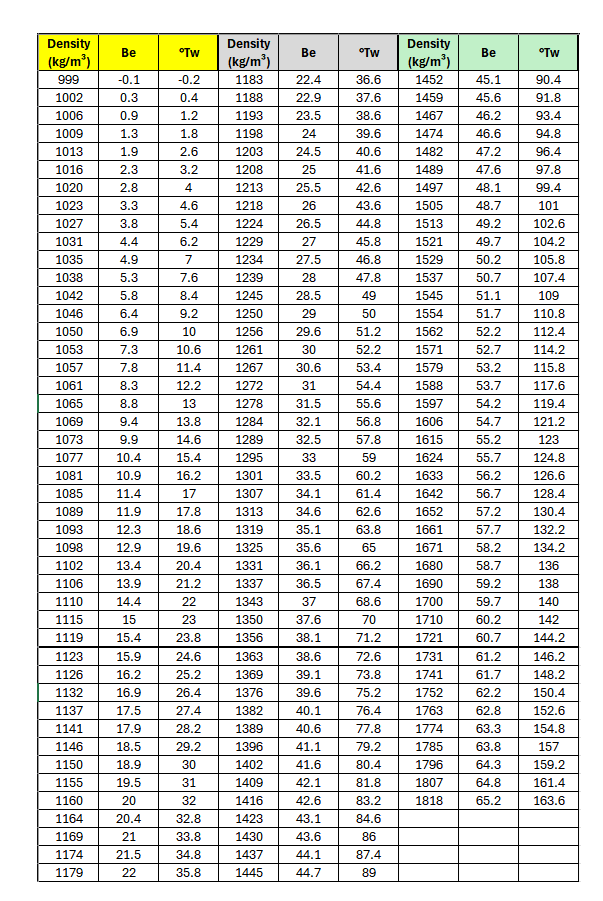
3.3 Density
The density of Soluble Silicates are usually determined by means of a hydrometer provided with a SI-units (kg/m³) scale. The following table helps to convert Baumé and Twaddle scale readings to SI-units.
The density depends on the concentration of solution, the temperature and the silica to alkali ratio. In practice, measurements must often be made at temperatures differing from the standard temperature of 20 °C. In such cases, the density obtained must be modified as follows: If the temperature of the solution is above 20 °C, the measured density must be increased by about 0.28 kg/m³ per 1 °C difference. The density must be decreased by the aforesaid value if the temperature is below 20 °C.
Comparison of Baumé and Twaddle scales with specific gravity
3.4 Viscosity
The viscosity is the most obvious property of Soluble Silicates. It is most important with regard to the handling and the application of the material.
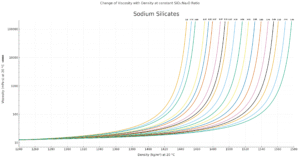
Many factors determine the viscosity of silicate solutions, the most important ones are silica to alkali ratio and concentration and temperature. The viscosity increases with rising concentration and ratio. It decreases with rising temperature. This interdependence is shown by various diagrams to be found in the supplement.
The addition of soluble salts to sodium and potassium silicate solutions generally causes an increase in viscosity. Mixtures of sodium and potassium silicate solutions usually show a higher viscosity than the basic solutions.
Viscometers:
A simple method to measure the viscosity bases on the determination of the rate of fall of a steel ball in a silicate solution. If the rate of fall is available, the viscosity of the solution expressed in terms of mPa·s can be easily calculated. The most satisfactory type of viscometer is the Hoeppler instrument using a ball rolling through the liquid in an inclined calibrated tube. All viscosity figures shown in this booklet were determined by means of a Hoeppler viscometer.
Other viscometers of Brookfield, Stormer or MacMichael type cleaned more easily than the Hoeppler instrument can be handled and results, too. Flow-out viscometers and instruments like the Redwood, Engler and Saybolt are not suitable for silicate solutions, nevertheless, they are sometimes used for an approximate estimation of viscosity.

3.5 Specific heat
As the viscosity of Soluble Silicates strongly depends on the temperature, very often storage tanks are to be provided with a heating system in order to achieve a solution which can be easily handled. For the determination of the required quantity of heat the specific heat of the silicate solutions can be taken into consideration as follows:
-
Sodium silicate 37/40: weight ratio 3.35 ≈ 2.97 kJ/kg·K
-
Sodium silicate 48/50: weight ratio 2.75 ≈ 2.51 kJ/kg·K
-
Potassium silicate 28/30: weight ratio 2.59 ≈ 2.97 kJ/kg·K
3.6 Melting point
Sodium and potassium silicates either in solution or as powders are often used as binders in coatings and cements for service at high temperatures. As the powdered silicates have varying softening properties at elevated temperatures depending on their nature and ratio, the selection of silicates is governed by temperature resistance required.
Sodium silicates become soft at temperatures of 590–670 °C and reach a fluid state at 760–870 °C. The softening point of potassium silicates is higher than that of sodium silicates.
3.7 Drying
The evaporation of Soluble Silicates at normal or elevated temperatures takes place initially at a fast rate and goes on with decreasing rate until further loss of water is negligible, although the product still contains water. The residual water persists for a long time at 100 °C or more above the boiling point of water. The elimination of this residual water requires heating at temperatures of 350–400 °C at least.
The drying and the water retention of sodium and potassium silicate solutions at a given temperature depend on the silica to alkali ratio. The higher the ratio, the faster the evaporation and the lower the residual water content.
3.8 Freezing
The behavior of Soluble Silicates at temperatures below 0 °C depends on their concentration and on their ratio SiO₂/Na₂O or SiO₂/K₂O.
For example, sodium silicate solution of 2.00 ratio SiO₂/Na₂O with 54% solids and 46% water keeps its vitreous appearance at temperatures far below –10 °C and returns to its initial state when being reheated.
A 3.30 ratio solution with 35% solids and 65% water at about –2 °C becomes opaque due to separation of ice crystals. These tend to float to the surface of the solution. When heated, the frozen solutions usually show layers of varying concentration due to the preceding separation of water as ice, which are very often irreversible.
3.9 Chemical reactivity
Dry films and coatings of sodium or potassium silicates will rehydrate and gradually dissolve in water. Therefore, heating of silicate films or bonds is not sufficient to obtain complete water resistance. This can only be achieved by chemical modification, such as by reaction with multivalent metal ions which all form insoluble silicates or by neutralization of the alkali with acidic reactants which cause that the silica is separated as a water resistant gel.
The acidic materials may be acids, acidic salts or organic compounds forming acids on hydrolysis in the alkaline medium. Soluble silicates can also be insolubilized by chemical reaction with amphoteric metal powders, basic metal oxides, alkaline aluminates, zincates, plumbates which all form insoluble metal silicates, mostly along with silica gel. Similar reactions can occur with powdered fillers and pigments.
Dried films of alkaline silicates absorb moisture and carbon dioxide from the atmosphere, lose their pristine vitreous character and develop a white efflorescence of alkali metal carbonate. Potassium silicates show less tendency toward efflorescence than the corresponding sodium silicates.
Sodium and potassium silicate solutions are incompatible with most organic compounds except sugar, polyols and urea. Mixing with aqueous dispersions of organic polymers usually produces coagulation of the organic colloid system as well as of the silicate solution. Alcohols, aldehydes, ketones, ammonia and salt solutions cause a certain salt out.
4.Analysis of Soluble Silicates
4.1 Titration of alkali
The alkali content of sodium and potassium silicate solutions is determined by titration of a sample of the material with standard acid and expressed as Na₂O or K₂O.
(Analysis photo caption: Analysis of soluble silicates by titration)
Procedure:
Dilute a weighed sample of 30–40 g sodium silicate solution or 50 g potassium silicate solution with distilled water to 500 ml exactly. Titrate 100 ml of the diluted solution corresponding to 6, 8 or 10 g silicate solution with 2 n hydrochloric acid, using methyl orange indicator solution.
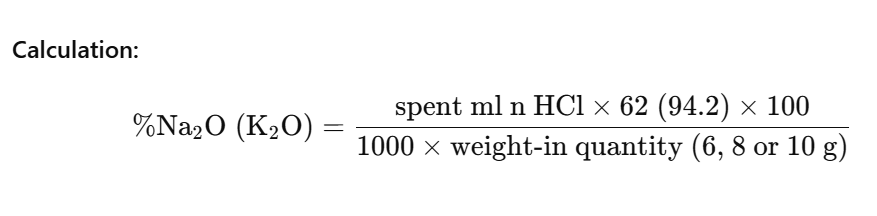
4.2 Titration of silica
The volumetric determination of silica is possible in the following way: The diluted silicate solution is first neutralized with acid. Then sodium fluoride is added in excess. The neutralized solution contains colloidal silica which reacts with the sodium fluoride as follows:
By this process the neutralized solution becomes alkaline again, and the formed alkali can be titrated with standard acids.
Much Experiences is required in order to get exact this volumetric method, but with practice a high degree of accuracy and considerable saving of time can be obtained .an operator should practice the method by determining silica on samples of known content for comparison.
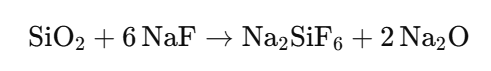
Procedure:
Transfer 50 ml of the diluted silicate solution corresponding to 3, 4 or 5 g silicate solution which was prepared for the titration of alkali into a 750 ml beaker, add 200 ml distilled water and neutralize the solution with 2 n hydrochloric acid using 5–8 drops methyl red indicator solution. The volume of acid required for this step must not be noted, but neutralization must be carried out accurately. Next add 10 g of sodium fluoride which has been exactly weighed in advance and stir well.
Titrate the obtained alkaline dispersion with 2 n hydrochloric acid, maintaining constant stirring. The end point is marked by an onion-skin-like brownish red colour. Thorough titration is necessary.
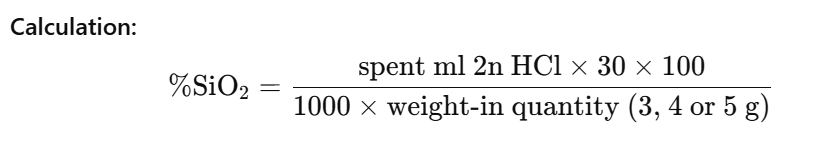
4.3 Gravimetric determination of silica
Silica is determined by precipitating a dilute silicate solution with hydrochloric acid and weighing the precipitate after washing, drying and ignition.
Procedure:
Transfer 25 ml of the diluted silicate solution corresponding to 1.5, 2.0 or 2.5 g silicate solution which was prepared for the titration of alkali into a porcelain evaporating dish and add about 25 ml of concentrated hydrochloric acid. Evaporate on a steam bath until all odor of HCl has disappeared. Thoroughly moisten the residue with 5 to 10 ml of concentrated hydrochloric acid and evaporate again to dryness. Repeat moistening and evaporating twice. Next treat the residue with 50 ml hot concentrated hydrochloric acid and add 50 ml of cold distilled water. Filter on a soft paper and wash with cold distilled water. Ignite the precipitate in a platinum crucible for 90 minutes at 1000 °C.
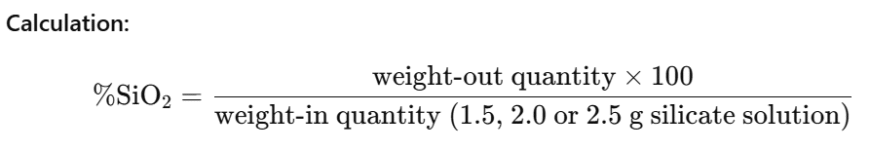
5.Packing, Transport and Storage of Soluble Silicates
5.1 Packing
The liquid silicates are supplied in non-returnable iron drums and road tankers.
The iron drums have the dimensions of approx. 58 × 89 cm, a capacity of 200 or 216 litres and contain 250 to 360 kg liquid silicate, the weight being determined by the density of the supplied grade. The tare is 18 kg or 23 kg. The drums have a M 64 × 4 mm bung in body and a R ¾” bung at the end.
It is recommended to store the drums inside in winter in order to prevent freezing of the liquid silicates.
The road tankers are semitrailer tank trucks having a maximum load of 20 tons. They are provided with a 6 m hose for connecting the trailer tank to the customer’s intake line. The road tankers are fitted out with a flange NW 80, ND 10 or a connecting piece TW 501 3″ and a transition piece NW 80, ND 10.
For unloading the tank truck an air compressor which works at maximum pressure of 1.5 bars and is capable of raising the less viscous grades to a height of 10 m in a reasonable time. If the store tank is below ground level, the silicate may flow by gravity. But in general, unloading is performed by a pump which should have a capacity of 300 to 350 litres per minute and must be installed by the customer.
5.2 Storage tanks
Silicate solutions may be stored in containers made from iron, steel or fiberglass-reinforced plastic. Occasionally reinforced concrete is used, but it is less satisfactory from an economical and technical point of view. Materials such as aluminium, galvanized iron, zinc and unsuitable. Highly alkaline silicate solutions and there are results as the linings of these must be insulated but the plastic storage tank as well as the glass are resistant to elevated temperatures.
The capacity of the storage tank is determined by the usage rate, the quantity of each consignment and the need to hold a sufficient stock to overcome potential delays in transport or increased requirements. Generally, the required capacity is calculated by means of the following formula: S=2R+T
Where: S = capacity of storage tank in tons; R = rate of consumption in tons per week; T = capacity of tank vehicle in tons.
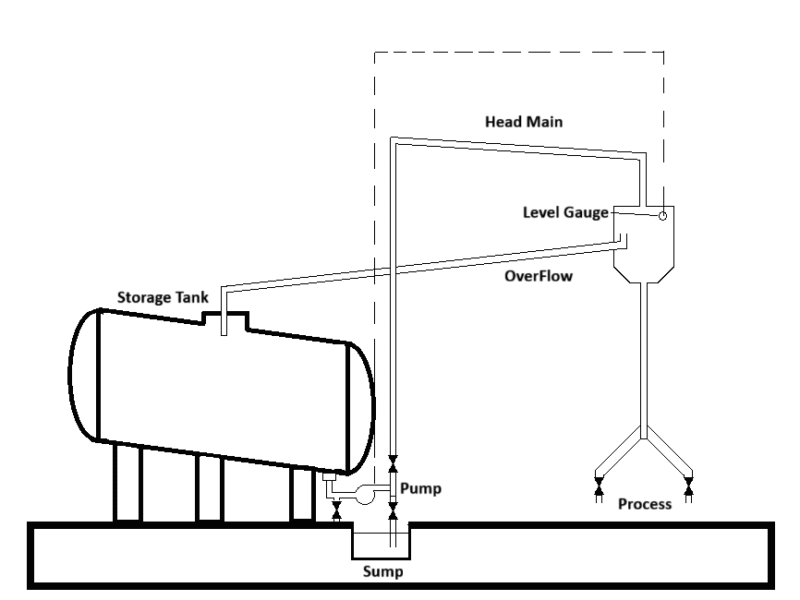
Typical storage and distribution systems are shown in the following figures.
Storage tanks should preferably be situated indoors to prevent freezing in winter. In this case a heating device on the storage tank is not necessary. Storage tanks which are situated in the open air must be insulated and should be provided with some means of heating. Local overheating should be avoided because of precipitation of silica.
Most commercial liquid silicates are not completely clear, the lack of clarity being caused by a very small amount of suspended silica and insoluble silicates. This suspended matter settles with time and results in some sludge on the bottom of storage tanks, especially if it was not thoroughly cleaned for a couple of years. The accumulation of the sediment can be minimized by positioning the take-off at the lowest point of the tank and by providing the storage tank with a rake in the direction of the take-off. In this way, any deposit will be carried away by the outgoing stream of liquid.
The storage tank should be covered and provided with a manhole and a bottom outlet for inspection, repairs and cleaning. The measuring of the solution level in the tank should preferably be effected by means of a float. The float should be attached to a wire which is taken over a pulley to the outside of the tank where it terminates in a weight freely hanging in front of a vertical graduated scale. An electrical alarm may be fitted, giving signals at certain filling heights.
5.3 Piping and valves
Ordinary black rough iron and steel pipes with a diameter of 2” to 3” are used for alkaline silicate solutions. Piping should be installed in such a manner that the system can be drained at one or more points. If the system is out of action, the pipes should be drained completely and cleaned, or they may stand filled with the silicate solution. Shutting clacks which are resistant to alkaline solutions are frequently used in silicate installations (Suppliers for Germany: GEFA, Dortmund; Keystone Armaturen, Grevenbroich).
5.4 Pumps
Centrifugal pumps made from iron or steel are recommended for the continuous pumping of the low viscous grades.When viscous grades of liquid silicates are to be transferred over a long distance or to a considerable height, and when pumping is intermittent, positive displacement pumps are required, e.g. diaphragm pumps
6.Safe handling of silicates
Sodium and potassium silicates are inorganic materials which neither decompose nor generate hazardous gases at high temperatures. All silicate solutions are, however, alkaline in nature and irritate skin and eyes.
In general, cold silicate solutions are unlikely to have any effect on the skin except the strongly alkaline grades. Extended contact should be avoided by wearing rubber gloves or rubber aprons. At all events, hot silicate solutions irritate or slightly burn the skin. In case of accidental contact, flush the area immediately with plenty of water.
Irritation or burning are likely if eyes are concerned. The strongly alkaline grades affect the eyes in the same way as sodium hydroxide solution. In case of contact with mildly alkaline grades serious complications are unlikely but in every case a strong irritation takes place.
All necessary precautions must be taken to avoid contact with the eyes. When handling silicate solutions, generally goggles should be worn. If silicate should splash into the eyes flush immediately with clean and if possible luke-warm water for at least 20–30 minutes and then get medical attention.
Spills of silicate solution should be cleaned up immediately with warm water to avoid accidents. Furthermore, the building up of glassy insoluble films can be avoided by this measurement.
7.Layout of the Head Tank Distribution System
8.Layout of the Loop Main Distribution System
9.Change of Viscosity with Density at Constant SiO2 : Na2O Ratios
10.Change of Viscosity with Density at Constant SiO2 : K2O Ratios